The global clinical trials market is predicted to be worth approximately USD 55.86 billion in 2023 and roughly USD 95 billion by 2030, with a strong CAGR of 7.07% between 2023 and 2030. Over the last decade, the worldwide clinical trials market has expanded due to a large increase in the number of clinical studies conducted around the world. The future of clinical research in India is moving toward patient-centeredness. Initiatives aiming at empowering and engaging patients in the trial process, along with a focus on customized care, have the potential to transform the clinical research landscape. Additionally, it was suggested that clinical teams could investigate various design options in “real time” with the help of algorithms programmed to deliver protocols. This would encourage the development of more intricate and creative study designs that are likely to yield valuable scientific and regulatory data
Importance of Clinical Research Course
The advancement of medicine and the enhancement of world health are significantly fueled by clinical research. Clinical research is the backbone of contemporary medicine, propelling breakthroughs and innovations in medicine that have the power to change lives. It is impossible to overestimate the importance of clinical research, which can be used to develop novel therapies or improve already effective ones. Demand for thorough and moral clinical research is only going to increase as more and more people—including patients, healthcare professionals, and pharmaceutical companies—realize how important evidence-based practice is. Understanding the value of clinical research is essential in today’s quickly changing medical environment to promote informed decision-making and influence healthcare’s future.
Why choose Clinical Research Course
Clinical research is an area that studies the efficacy and safety of new medical treatments, technologies, and procedures. It is a major component of the healthcare business, contributing significantly to the discovery of novel pharmaceuticals and therapies that can enhance patients’ lives. If you are interested in working in healthcare or want to make a difference in people’s lives, clinical research may be a good fit for you. In this blog post, we will look at why you should pursue a career in clinical research. Conducting research studies can aid in the development of new medications, treatments, and technology that improve patient outcomes and quality of life. Every innovation in clinical research has the potential to significantly improve the lives of patients, and working in this field allows you to be a part of that effect.
Career Growth Clinical Research Course
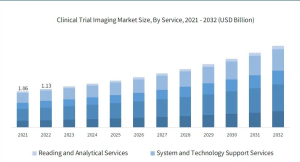 Clinical research is the foundation of medical advances. The goal is to test new medications, therapies, and medical technologies on humans to verify they are safe, effective, and beneficial to patients. It’s a thorough procedure, from trial design to data collection and analysis. India’s clinical research scene is thriving! The business is expected to reach a stunning USD 7.1 billion by 2027, with a 29% annual growth rate, making it a magnet for job prospects. Over 127 CROs operate in India, performing trials for 60% of all pharmaceuticals in development. Every year, 51,000 new employment are produced, giving life science graduates the opportunity to be a part of this flourishing landscape. Buckle up, because it’s going to be an entertaining ride.
Clinical research is the foundation of medical advances. The goal is to test new medications, therapies, and medical technologies on humans to verify they are safe, effective, and beneficial to patients. It’s a thorough procedure, from trial design to data collection and analysis. India’s clinical research scene is thriving! The business is expected to reach a stunning USD 7.1 billion by 2027, with a 29% annual growth rate, making it a magnet for job prospects. Over 127 CROs operate in India, performing trials for 60% of all pharmaceuticals in development. Every year, 51,000 new employment are produced, giving life science graduates the opportunity to be a part of this flourishing landscape. Buckle up, because it’s going to be an entertaining ride.
.
CURRICULUM
Introduction to Clinical Operations
- Role of Clinical Operations
- Clinical Trial Phases
- Regulatory Framework
- Key Stakeholders
- Clinical Operations Team
Study Planning and Initiation
- Feasibility Studies
- Budgeting and Resource Allocation
- Site Selection and Activation
- Investigator Meeting Coordination
- Regulatory Document Preparation
Clinical Trial Management
- Project Management
- Site Management
- Monitoring and Quality Control
- Risk Management
- Data Management Coordination
Study Close-Out and Reporting
- Study Close-Out Procedures
- Data Cleaning and Lock
- Final Report Preparation
- Regulatory Submissions and Inspections
- Post-Trial Activities





