The future of clinical SAS programming will see even more integration with data standards, assuring seamless compliance and promoting smoother interactions among many players in the clinical research ecosystem. Due to the tremendous growth in clinical trials, major pharmaceutical corporations and CROs are boosting their outsourcing from India. The clinical trials market is currently worth $49.8 billion and is expected to increase to $78.3 billion by 2030.Let us continue to communicate, share knowledge, and adapt to the changing world of clinical SAS programming. The future is bright, and together, we will open up new opportunities and build the future of data-driven healthcare.
Importance of Clinical SAS
SAS certification offers an organized path to professional growth. As people advance through the certification levels, they develop deeper insights and skills in SAS, which can lead to more advanced roles and responsibilities within organizations. Clinical SAS has extensive data management tools that allow researchers to easily handle enormous datasets. Its capacity to integrate and transform data from several sources provides data correctness and consistency, which is critical for generating solid results in clinical research.
Why choose Clinical SAS
Clinical SAS’s statistical analytic capabilities are one of its key strengths. The program provides a comprehensive set of statistical techniques and algorithms for analyzing complex clinical trial data. From fundamental descriptive statistics to complex modeling techniques, Clinical SAS enables researchers to draw relevant insights and make statistically sound conclusions. Furthermore, it facilitates the preparation of detailed and regulatory-compliant reports, which are required for submissions to regulatory agencies.
Career Growth Clinical SAS
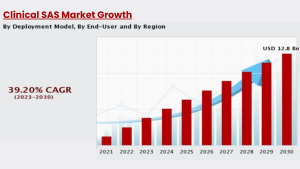 As a Clinical SAS professional, you can anticipate a diverse range of employment prospects. Clinical SAS Programmer and Data Analyst are common entry-level occupations. These jobs entail working with raw data from clinical trials, preparing it for analysis, and producing summary statistics. Individuals with Clinical SAS knowledge and proficiency can rise to jobs such as Clinical Data Manager, Biostatistician, or even Clinical SAS Consultant, providing expertise to numerous firms. Due to the huge growth in clinical trials, major pharmaceutical corporations and CROs are boosting their outsourcing from India. The clinical trials market is currently worth $49.8 billion and is expected to increase to $78.3 billion by 2030.
As a Clinical SAS professional, you can anticipate a diverse range of employment prospects. Clinical SAS Programmer and Data Analyst are common entry-level occupations. These jobs entail working with raw data from clinical trials, preparing it for analysis, and producing summary statistics. Individuals with Clinical SAS knowledge and proficiency can rise to jobs such as Clinical Data Manager, Biostatistician, or even Clinical SAS Consultant, providing expertise to numerous firms. Due to the huge growth in clinical trials, major pharmaceutical corporations and CROs are boosting their outsourcing from India. The clinical trials market is currently worth $49.8 billion and is expected to increase to $78.3 billion by 2030.
CURRICULUM
Introduction to SAS Programming in Clinical Research
- Overview of SAS Software
- Basic SAS Programming Concepts
- Clinical Trial Data Structures
- Regulatory Guidelines
- Role of SAS Programmers
Data Management and Manipulation with SAS
- Importing and Exporting Data
- Data Cleaning and Validation
- Data Merging and Appending
- Creating Derived Datasets
- Using SAS Functions
Statistical Analysis with SAS
- Descriptive Statistics
- Inferential Statistics
- ANOVA and Regression Analysis
- Survival Analysis
- Interpreting Output
Clinical Reporting and CDISC Standards
- Generating Tables, Listings, and Figures (TLFs)
- Adhering to CDISC Standards
- Creating Define.xml
- Quality Control and Validation
- Preparing for Regulatory Submissions






