The global pharmacovigilance market was valued at USD 6.33 billion in 2020 and is predicted to increase at an 11.5% compound annual growth rate (CAGR) between 2021 and 2028. We may witness a substantial change in the pharmacovigilance system worldwide due to encroachments in technology, hence expanding the availability of data to corporations as well as regulators. The challenges include the fact that studies are typically conducted on a small number of patients, chosen based on stringent eligibility criteria that may not accurately represent the actual population, and have a shorter duration, posing a barrier to long-term identification of adverse reactions.
Importance of Pharmacovigilance
Pharmacovigilance is critical for maintaining public health and drug safety. That is why pharmaceutical companies must create an effective and foolproof mechanism for evaluating the undesirable effects of drugs. Pharmaceutical manufacturers must be well-versed in pharmacovigilance recommendations. Pharmacovigilance is the science and practice of detecting, assessing, understanding, and preventing side effects or other medical/vaccine-related problems. Before being approved for use, all medications and vaccines are subjected to rigorous safety and efficacy testing in clinical trials.
Why choose Pharmacovigilance
In a fast-paced and continuously changing industry like healthcare, rigorous medication safety monitoring has become critical. Here is where pharmacovigilance comes into play. As pharmaceuticals become more complicated and diversified, it becomes increasingly important to ensure their safety and effectiveness. And if you’re thinking about a career in the UK, pharmacovigilance could be the perfect fit. There is an increasing demand for experienced people in pharmacovigilance. This sector provides a unique chance to contribute to public health by finding, evaluating, and preventing drug-related side effects. Not only will you be at the forefront of patient safety, but you will also have the opportunity to collaborate with top pharmaceutical firms, regulatory agencies, and healthcare organizations.
Career Growth in Pharmacovigilance
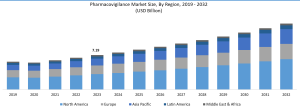 Entry-level roles in the pharmaceutical business are an excellent opportunity to begin your career in pharmacovigilance. Jobs in pharmacovigilance often require a degree in life science, pharmacy, or a related profession. Most pharmacovigilance career entry levels include drug safety specialists, drug safety associates, and drug safety coordinators. These responsibilities are primarily responsible for accessing and monitoring adverse drug reactions, as well as database maintenance, the preparation of safety reports, and maintaining regulatory compliance. Pharmacovigilance In 2022, the market size exceeded USD 8.5 billion. Driven by the global demand for novel pharmaceuticals, the market is predicted to rise at a CAGR of more than 8.5% from 2023 to 2032.
Entry-level roles in the pharmaceutical business are an excellent opportunity to begin your career in pharmacovigilance. Jobs in pharmacovigilance often require a degree in life science, pharmacy, or a related profession. Most pharmacovigilance career entry levels include drug safety specialists, drug safety associates, and drug safety coordinators. These responsibilities are primarily responsible for accessing and monitoring adverse drug reactions, as well as database maintenance, the preparation of safety reports, and maintaining regulatory compliance. Pharmacovigilance In 2022, the market size exceeded USD 8.5 billion. Driven by the global demand for novel pharmaceuticals, the market is predicted to rise at a CAGR of more than 8.5% from 2023 to 2032.
CURRICULUM
Introduction to Pharmacovigilance
- Overview of Pharmacovigilance
- History and Evolution
- Regulatory Framework
- Roles and Responsibilities
- Key Terminologies
Adverse Event Reporting and Management
- Types of Adverse Events
- AE Reporting Systems
- Case Processing and Management
- Risk Assessment and Mitigation
- Regulatory Reporting Requirements
Signal Detection and Risk Management
- Signal Detection Methods
- Data Mining and Statistical Tools
- Risk Management Plans (RMPs)
- Periodic Safety Update Reports (PSURs)
- Benefit-Risk Assessment
Pharmacovigilance in Clinical Trials and Post-Marketing
- Pharmacovigilance in Clinical Trials
- Post-Marketing Surveillance
- Pharmacovigilance Audits and Inspections
- Safety Communication
- Pharmacovigilance Systems and Compliance







